Adopting a structured and streamlined approach to medical writing can help increase efficiency when responding to authority questions in the marketing approval process.
A great amount of effort goes into preparing a submission dossier to apply for marketing approval of a medicinal compound. However, process doesn’t stop once the dossier goes out the door. In fact, sometimes, that is when the really tricky part begins. After receipt and review of the dossier, the authorities respond to the sponsor with their questions, which usually address the weaknesses or sticking points of a development programme. At this point the team faces the biggest challenge of all: writing and coordinating a set of responses to these questions that make the science clear and demonstrate to the reviewers that the risk benefit- assessment is well-founded.
REVIEW TIMELINES
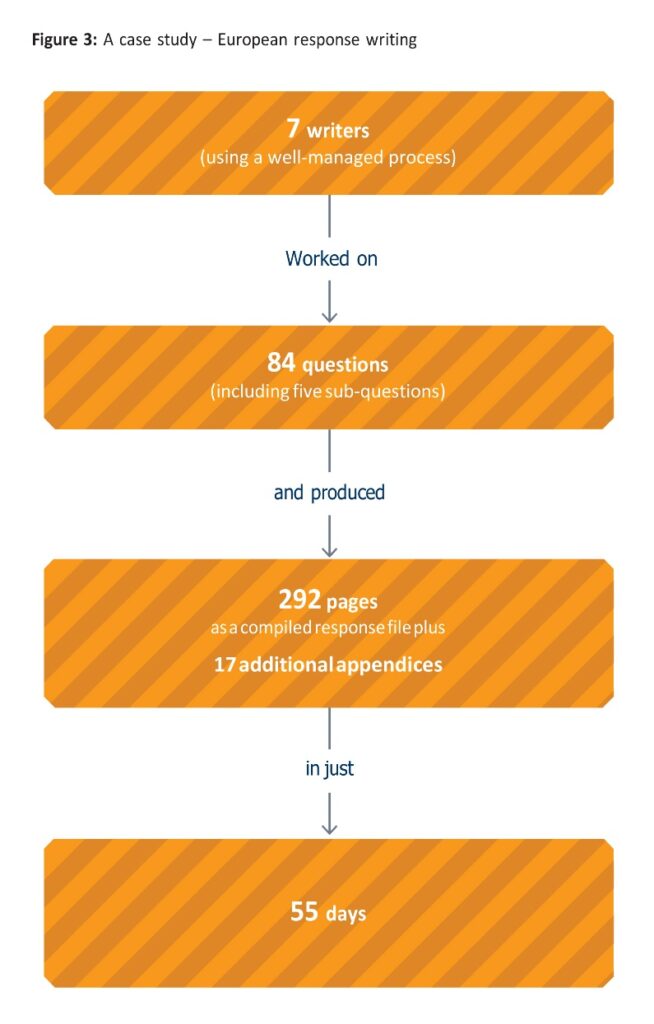
To adequately plan for and streamline activities associated with preparing responses, the regional variability in how questions are posed to the applicant need to be considered. For a European centralised procedure, the review process follows a strictly defined timeline [1]. Review begins on day one (clock start), and the initial assessment report from the rapporteur is sent to the Committee for Medicinal Products for Human Use (CHMP) and to the sponsor on day 80. The assessment report includes a preliminary list of questions and provides the first insight to the reviewers concerns regarding the dossier. A consolidated and final list of questions is sent to the sponsor on day 120 from the CHMP (clock stop). Applicants then have three months to respond to the questions [2]. An additional period of up to three months can be requested if there is appropriate scientific justification, but extensions beyond six months are not normally accepted. With such a tightly defined process, the timing of questions is predictable and, because a set of preliminary questions is shared with the applicant on day 80, the applicant has an advance warning period of 40 days to address key questions before the start of the three-month response period. This makes it easy to plan resources and offers more time to develop a strategy for answering the questions.
The timeline for review is more loosely defined after submission of a drug application dossier to the Food and Drug Administration (FDA) in the US. The FDA reviewer can ask questions at any time until the end of the review period, which may be at six or 10 months, during which time the applicant must be available to respond to any questions as quickly as possible. Because the timing of questions is not predictable, it can be more of a challenge to plan resources and puts pressure on the teams to determine their strategy for response in less time.
A number of techniques can help coordinate and manage the challenge of answering these questions (see Figure 1). The first is to get ready in advance. This consists of two steps: anticipating potential questions and then planning your response team.
It is helpful to start planning for potential questions while writing the dossier. There are usually a handful of obvious questions the authorities will want to explore in detail for every development programme: those gaps in the data, problems that arose while running the studies, differences of opinion about analysis methods or clinical practice, and so on, that become obvious while the drafts of the summaries are being written and reviewed internally. Making a list of all these issues is useful because it is likely that at least half of these appear as questions from the regulatory reviewers. Armed with this list, after the dossier has been sent off, the response team can focus on coming up with creative ways to explain each of these issues in more detail than they may have done in the dossier itself.
PLANNING THE RESPONSE TEAM
It is vital to ensure that the people required to answer a particular question will be available when the time comes. It is not uncommon for people to take vacations after the intense efforts of preparing a submission dossier, and this can cause problems if their input is needed quickly to answer a question. Someone from each function must be available at the expected times to answer any potential questions. So make a list of each functional area that could be needed, then assign a person who has primary responsibility for that area, as well as a backup person. Identify where these people will be during the expected response writing period and make sure they are aware of the times they need to be available. By communicating this in advance to everyone who is needed, unpleasant surprises can be avoided, such as a major concern being raised about a pharmacokinetic issue and realising your pharmacokineticist is in the tropics without a mobile phone connection.
Once questions begin to arrive, it is extremely helpful to prepare a tracking sheet to monitor the status of each question. Because the lead medical writer oversees the preparation of the documents, they are ideally positioned to prepare and update this list as the writing activities proceed. It is important to be able to quickly identify the status of each question, such as which questions are only in a first draft, still need a quality control check, which are waiting for the outcome of further analyses, or which are complete. Because work usually takes place in parallel on numerous responses, and these responses can sometimes be small reports unto themselves, the process is much more efficient if someone has an overview of what stage each response is at.
Ideally, preparations for writing the responses should begin with a brainstorming session with the entire response team from a given area – for example, all of the contributors from the clinical team. While walking through each of the questions, the lead medical writer will make a bulleted list outlining what the team thinks are the key messages or tactics to answer each question. The team needs to identify if new analyses will need to be performed to adequately answer the question (which should be captured in the tracking sheet). Make sure that in addition to the writer, there are one or two people assigned to each question to provide the content expertise from the corresponding functional areas (such as statistics or pharmacovigilance). If possible, adopt a divide and conquer approach to spread out the burden among team members, keeping in mind that time is short and the more the workload can be distributed, the faster first drafts can be prepared.
Next, identify who will prepare the first draft for each response; the medical writer or a contributing author. For many responses, if the strategy is clear, it can be quite straightforward for the medical writer who worked on the dossier to pull together the required information and craft an initial text. However, if a more in-depth understanding of a particular area is needed, it might be more effective to have the responsible person craft an initial text (a clinician or statistician, for example), which the medical writer can later refine and make consistent stylistically with the other response texts.
The lead writer should also ensure the team discusses and agrees on the review process, including setting binding timelines for when drafts are due and when review feedback is to be provided. It should be clear exactly who needs to review the response to which questions, and it is a good idea to put this information in the tracking sheet, so that it doesn’t get forgotten or overlooked. Will each response be reviewed once or twice? At which stage should it go to broader team review? Who is going to consolidate the feedback from each round of review? All these questions should be clarified in advance to avoid misunderstandings and make sure everyone knows what they need to do and when.
Ideally, each response should initially be written as a separate Word file. Although the responses will ultimately be pulled together into a consolidated document at the end, until each response gets to a final draft stage, it is much more effective to handle them separately from each other. Keeping each response as a separate entity until the end enables each one to be written and reviewed in isolation, so that work can continue on all others in parallel.
COMMUNICATION AND AGREEMENT
The practical logistics of working with individual files for each response means, however, that responses need to be organised in the context of the final consolidated file of responses. This means the writing team needs to agree at the start on factors such as how to implement cross-referencing between responses or consistent use of abbreviations. Defining these things in advance saves a lot of headaches when it comes to compiling the final responses into a unified document.
Regular meetings with the response team are important to make sure everyone knows what others are doing and in order to prioritise activities. Use the tracking sheet to drive these meetings, updating the status of each response, identifying critical path activities (such as new activities that arise including the need for new analyses) and defining who is responsible for them.
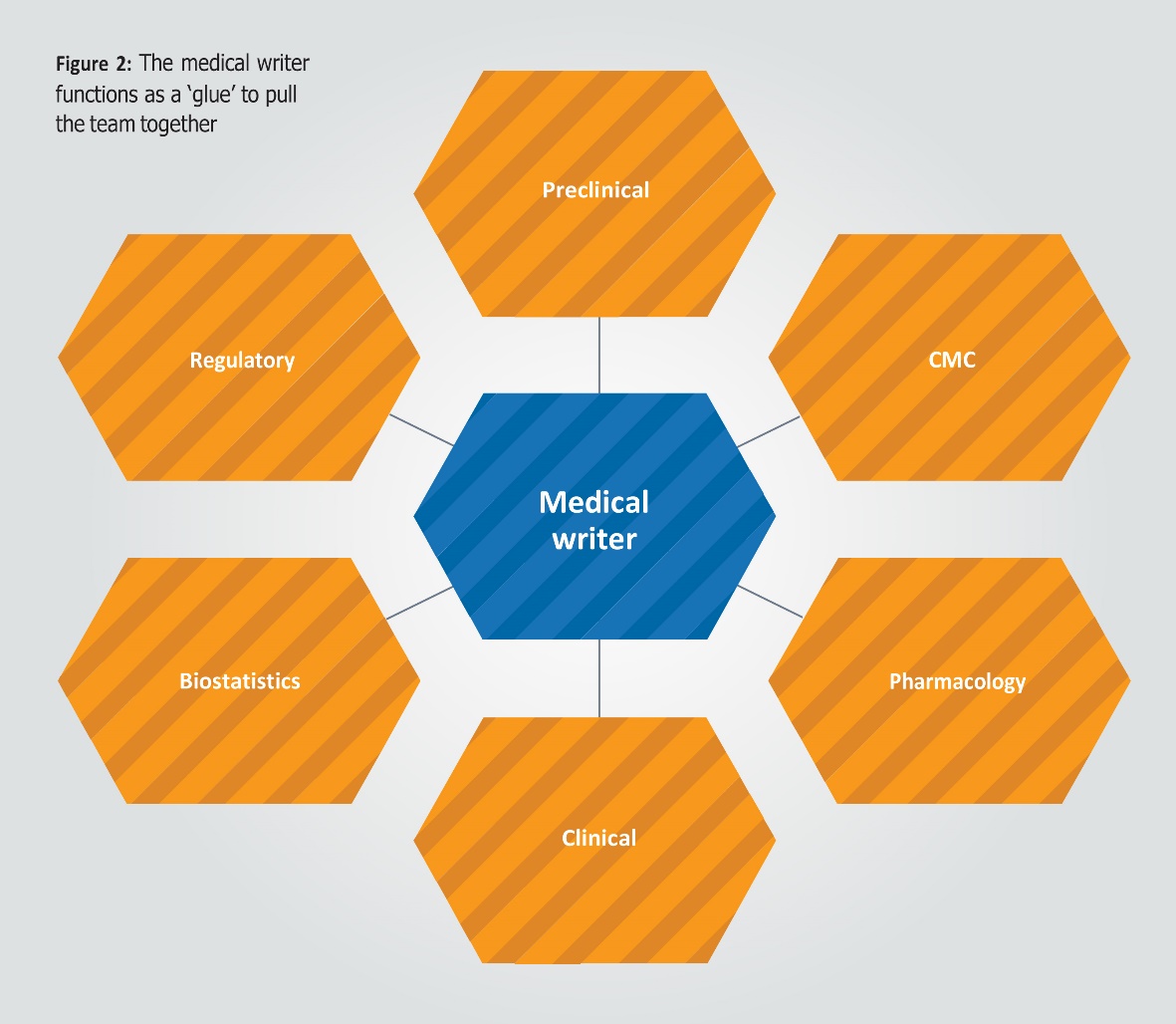
There is a lot of activity happening in a short time frame, with anywhere from 10 to 100 different response documents being developed and reviewed simultaneously, so the lead medical writer sits at the eye of the storm, and holds the team together in these periods. They pull together input from different functions, coordinate parallel review cycles and meetings to discuss the outcome of these, and maintain an overview of progress by constantly updating the tracking sheet (see Figure 2). By understanding the role they play and setting up the process to enable them to apply their tricks of the trade, the whole experience can be simplified and made much more efficient for all involved.
CONCLUSION
Preparing responses to authority questions is often similar to running a relay race. Numerous responses are written in parallel, with several documents at different stages of completion, all being passed among team members to review in a very short time frame. And it is important that none of the documents fall between the cracks and get left behind in the process. With the assistance of an experienced medical writer, the process of writing and coordinating responses to authority questions can be made as efficient as possible and, ultimately, reduce the stress often associated with this activity.
REFERENCES
- Article 6(3) of Regulation (EC) No. 726/2004 of the European parliament and of the council of 31 March 2004, Laying down community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency
- European Medicines Agency, Time allowed for applicants to respond to questions and issues raised during the assessment of new marketing authorisation applications in the centralised procedure, Doc Ref EMEA/75401/2006, Rev 2
Julia Forjanic Klapproth
Published in: European Pharmaceutical Contractor, March 2012