There is no doubt that the public interest in healthcare-related issues is growing. This, coupled with the surge in the use of social media, leaves the pharmaceutical industry with a set of unique opportunities and challenges. The screening and reporting of adverse drug reactions (ADRs) is of vital importance, and Marketing Authorisation Holders (MAHs) have a responsibility and liability for their drugs. Patients increasingly use social media to share their healthcare experiences, and this is a welcome opportunity for MAHs to learn more about the real-world experience of their products. However, currently this source for ADR reporting is largely underutilised; partly because the data generated are unstructured, but also because our technology for assessing and analysing this information is lagging behind. There is an urgent need for policy, methods, guidelines, and technology platforms to allow patients’ voices through social media to be adequately ‘heard’ and incorporated into the benefit-risk assessment of drugs.
The information gathered through digital media is increasing exponentially, especially through the data contributed by social media. Additionally, the majority of the data responsible for the exponential growth of knowledge are unstructured, and include tweets, comments on social media sites such as Facebook, and videos posted on websites such as YouTube. While the public’s interest in dis- cussing healthcare-related issues is growing, our technology for assessing and dealing with this type of information is struggling to keep up. Although marketing departments of pharmaceutical companies have already begun using social media to understand the perceptions of patients about their drugs, other departments – such as safety and pharmacovigilance – are still sceptical about the validity of the knowledge extracted from social media.
SHOULD WE BE INTERESTED IN SOCIAL MEDIA REPORTS OF ADVERSE DRUG REACTIONS?
The European Medicines Agency’s (EMA) Good Pharmacovigilance Practices (GVP) defines an adverse drug reaction (ADR) as a response to a medicinal product which is noxious and unintended. ADRs are considered as serious if they involve death, a life-threatening condition, inpatient hospitalisation or prolongation of hospitalisation, persistent or significant disability or incapacity, a congenital anomaly, or a birth defect. An individual case safety report (ICSR) describes one or several ADRs that occur in a single patient at a specific point in time.
The criteria for an ICSR to be valid include:
- at least one identifiable reporter,
- a single identifiable patient,
- at least one suspect adverse reaction, and
- at least one suspect medicinal product.
It is challenging to apply these definitions to social media reports of ADRs, but the importance of ADR reporting cannot be overestimated. The continuous surveillance of the safety and efficacy of pharmaceutical products used in clinical practice helps the early detection of drug safety problems in patients and, thus, serves to reduce drug morbidity and drug mortality.[1] ADRs can also have negative effects on treatment adherence and, consequently, increase the risk of resistance and disease. Furthermore, the treatment of ADRs incurs additional healthcare expenses due to hospitalisation or other medical interventions.[2] Therefore, the screening and reporting of ADRs is of vital importance.
Marketing Authorisation Holders (MAHs) are legally responsible for the safety and effectiveness of medicines on the market, and are required to report ADRs to the national pharmacovigilance centre, a medicines regulatory authority, or the World Health Organization, as appropriate.[2]
MAHs are required to operate appropriate pharmacovigilance and risk management systems to ensure responsibility and liability for marketed products and to ensure that appropriate action can be taken when necessary. To be able to fulfil this requirement, the MAH must have a thorough understanding of the ADRs caused by their products.According to EMA GVP Module VI MAHs should regularly screen internet or digital media under their management or responsibility for potential reports of suspected adverse reactions. If the MAH becomes aware of a report in any non- company sponsored digital medium, the report should still be assessed to determine whether it qualifies for reporting. Within the European Union MAHs are legally obliged to forward adverse events (AEs) to the EMA. In addition, there are other voluntary programmes in place to improve ADR reporting, e.g. the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS)[3] and The Yellow Card Scheme in the UK.[4]
However, given that patients are increasingly using social media to post and discuss their experiences with diseases, drugs, and treatments, a vast amount of data is now generated specifically concerning reactions to medicines that MAHs are required to monitor, assess, and act on, as appropriate. Current tools and techniques for the analysis of unstructured data, especially in the domain of pharmacoepidemiology and safety, are very limited. Also, discussions about the validity and reliability of data traditionally have excluded unstructured data provided by patients from the range of acceptable ‘evidence’ in the evaluation of the benefit-risk balance of medicinal products. Consequently, very few studies have been conducted on the extraction of knowledge from these sources, and there are methodological and technological gaps in this area which must be filled.
WHAT MAKES DIGITAL MEDIA AN ATTRACTIVE SOURCE OF KNOWLEDGE ON HEALTHCARE?
Digital media refers to audio, video, and images that exist in a computer-readable format and includes web- sites, web pages, blogs, vlogs, social networking sites, internet forums, chat rooms, and health portals. To this list, one should also add the ‘internet of things’ which is the interconnection of uniquely identifiable embedded computing devices within the existing inter- net infrastructure (e.g. GPS devices, cars, cameras, ‘coach’ watches, sphygmomanometers, glucose measuring devices, insulin pumps, bathroom balances).
An unprecedented volume of information now exists on these media, with the number of social media users growing phenomenally over the past few years. For example, the number of registered Google Plus active users increased from 500 million to 1 billion within a period of less than a year in 2012.[5] It is estimated that an individual who is already an active user of social media spends around 13 to 16 minutes per hour on social media websites,[6] and may engage in collaborative projects (e.g. Wikipedia), share information on social networking websites (e.g. Facebook), participate in virtual games and social worlds (e.g. World of Warcraft, Second Life), or create and share videos (e.g. on YouTube, Vimeo).
It is estimated that these websites will increase the amount of recorded data to 44 zettabytes by 2020 (1 zettabyte = 1021 bytes) as compared to 1 zettabyte in 2010. This is a massive amount of unstructured data compared with the estimated 5 zettabytes of data from structured sources by 2020. It is estimated that 9% of all unstructured data will be related to healthcare, of which half will be related to drugs.[7] Internet-based applications are also easily accessible and within the reach of large groups of people, and thus can be updated immediately and with high frequency.
VALIDITY AND USEFULNESS OF PATIENT POSTINGS IN SOCIAL MEDIA
Traditionally, AE reporting relies on physicians and drug safety groups, who serve as gatekeepers to validate the reports. Patients are increasingly using social media to share their experiences with drugs, medical devices, and vaccines.[8] However, currently this source for ADR reporting and pharmacovigilance is largely underutilised. To date, there are only five articles on PubMed that discuss tracking ADRs with the help of social media.
In a study described in one of these articles, Freifeld and colleagues identified more than 4000 posts on Twitter that resembled AEs and showed that they were significantly correlated with data from FAERS by System Organ Class (P < 0.0001).[8] Internet data on ADRs from consumers may also serve to identify areas for service improvement or topics about which patients need more education or information. For example, analysis of 3785 items from five social media sites found that patients with glaucoma had stronger positive feelings towards complementary therapies and treatments with a poor evidence base than towards medically proven therapies,[9] suggesting a lack of awareness or education about the clinically proven treatments.
Web-based patient-reported outcomes can provide an opportunity for MAHs and regulatory bodies to understand the benefits and risks of medicines in the real world. However, there are concerns about the validity of data from social media sites. Guidelines developed in consultation with industry, patients, regulators, academic groups, and prescribers are urgently needed to suggest methodologies to collect, analyse, and process this large pool of information,[10] which could potentially help in early detection of unrecognised side effects. Such methodologies and may serve as complimentary tools for MAHs and the FDA for receiving patient feedback.[11]
In addition, knowledge of social media discussions will help physicians to better understand how patients perceive their ADRs and to manage the adverse effects of drugs and develop strategies for improving treatment adherence. A mixed methods study examined the content related to aromatase inhibitor (AI)-associated side effects posted by breast cancer survivors on 12 message boards between 2002 and 2010. Of the 25,256 posts related to AIs, 18.2% mentioned at least one side effect. Furthermore, 12.8% mentioned discontinuing AIs and 28.1% mentioned switching AIs.[12]
Online communities may also highlight topics that are of concern to patients (e.g. medication convenience or packaging) and side effects that are not discernible in clinical trials.[13] In addition to ADRs, social media data can also help assessment of the risk perceptions of patients. Analysis of patient narratives on popular social media websites for health-related topics in France before and after withdrawal of all medicines containing benfluorex found that there was drastic change in the patients’ perceptions after withdrawal. Prior to the withdrawal date, most posts concerned efficacy, while those after the withdrawal date dis- cussed cardiovascular side effects.[14]
DO WE HAVE THE TECHNOLOGY FOR TAPPING THESE UNSTRUCTURED DATA?
The studies described above are small scale and ‘one shot’ examples. Given the large volume of unstructured data available on the internet, an efficient data solution is needed to detect ADR reports on social media and bring them to the attention of MAHs for validation and further actions.
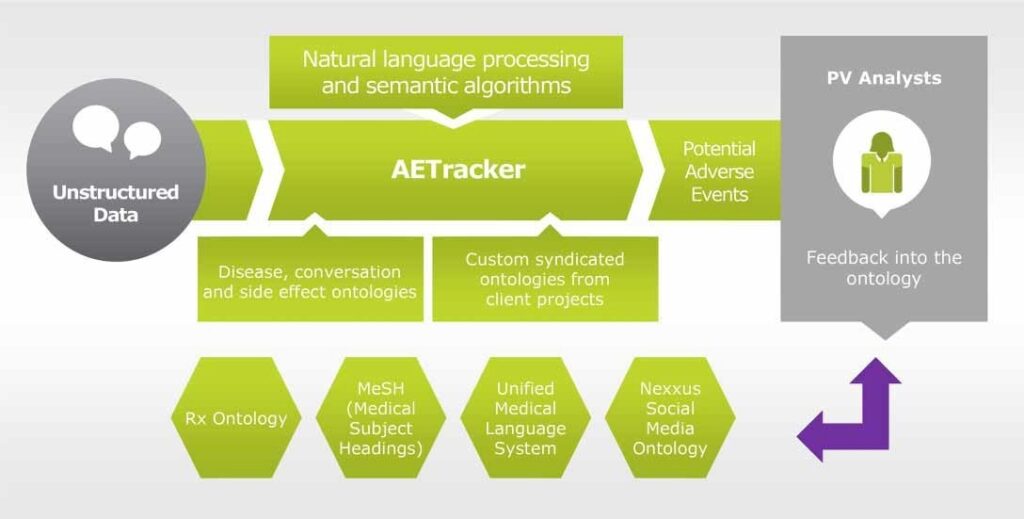
Few examples exist today which could provide insights into the technical possibilities of detecting safety signals through the internet. One such example is IMS Health’s Nexxus™ Application Suite and its module AETracker. This module provides a cloud-based engine for AE monitoring, off- label usage, and other legal, regulatory, and reputation risks in company-sponsored digital assets including social media accounts and mobile apps. In real time, pharmacovigilance experts review and confirm any false positives or alert the client within 1 hour of an AE being reported (Figure 1).
Figure 1: AETracker: A solution for ADR tracking on the social media.
In a test project, the system processed websites such as Twitter, Facebook, forums, and Wikipedia pages and generated 281,971 records related to a specific drug, of which 15% were flagged as potentially interesting signals by AETracker and 1.7% were confirmed following analysis. Notably, the system flagged every record that looked like an AE with 100% accuracy. This approach showed that technological advances such as the Hadoop system, natural language processing, and logical programming could provide useful methods allowing robust analysis of internet-based safety signals. Also, it showed that MAHs could save 85% of costs related to pharmacovigilance as compared to a moderation approach.
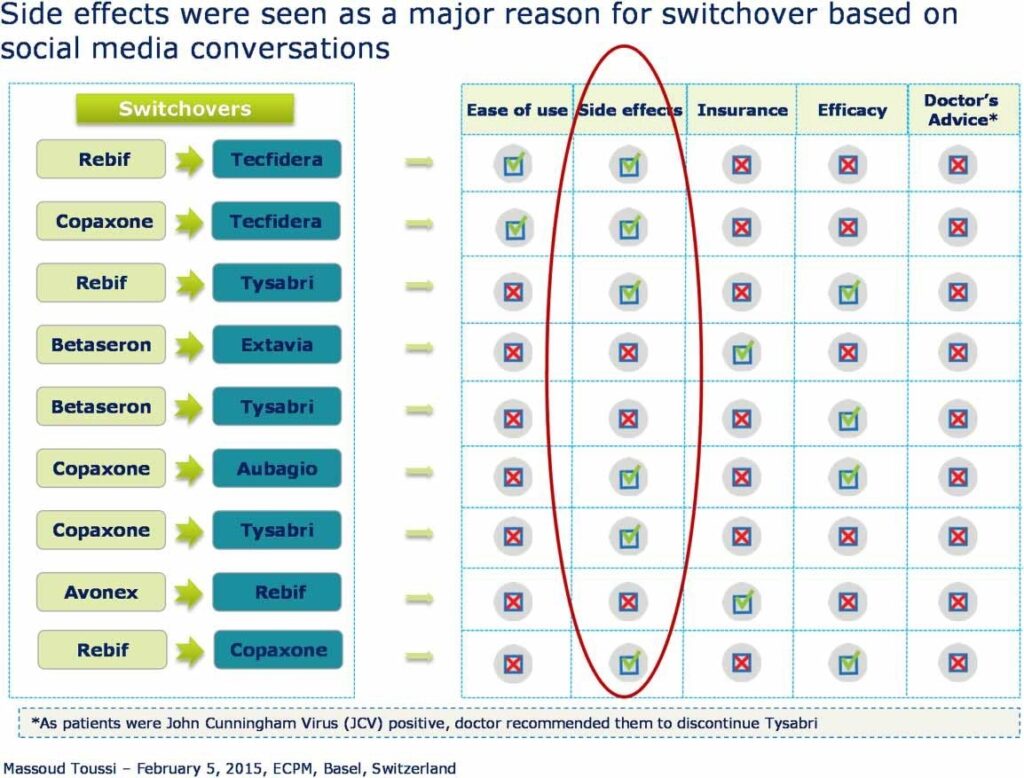
Beyond the interest related to the detection of safety signals, such tools also allow MAHs and healthcare professionals to gain a better understanding of the information exchange about a drug, and to better understand patient and consumer perceptions about that drug. For example, the use of AETracker resulted in an observation that Copaxone is perceived to be a highly effective first-line injectable for multiple sclerosis, followed by Rebif, Avonex, and Betaseron, in decreasing order of efficacy. Betaseron and Betaferon were perceived to be easier to use and associated with fewer side effects (e.g. injection site reactions) than Rebif and Avonex. Extavia and Avonex were perceived to be more cost effective than Rebif, Copaxone, and interferon β-1b drugs. According to social media conversations, side effects were the main cause for patients to switch treatments (unpublished observations, Figure 2).
Figure 2: Summary of social media conversations on the use of injectable drugs in multiple sclerosis.
In the UK, the WEB-RADR initiative is being led by the MHRA. This initiative seeks to investigate technologies for gathering ADR data, and will develop tools and recommend policy. WEB-RADR is a multi-stakeholder initiative and comprises the development of a mobile app for collecting ADR data. The data collected via this app should add information to the established safety profiles of medicines, enable earlier detection of new signals, reveal new patterns or trends in reporting, and even provide a means for geo-pharmacovigilance.
Technologies in development such as WEB-RADR will allow patients to report ADRs through apps or social media sites. Ideally, these reports would be analysed alongside other sources of pharmacovigilance data, and any signals identified and regulatory action agreed. Feedback can also be provided directly to the patient, but this two-way communication should be handled with care because of ethical and legal implications. Data gleaned from social media are also subject to validation, assessment, and duplication issues. Currently, collaboration between IMS Health and Facebook allows de- duplication of the majority of reports in AETracker.
CONCLUSION
There is no doubt that ADR reporting methodologies are changing and that the amount of data generated through social media is rapidly growing. This provides an opportunity for MAHs and healthcare professionals as information on ADRs shared on social media can be used as a source of information and insights for the benefit-risk evaluation of drugs.
There is an unmet need for policy, methods, guide- lines, and technology platforms describing the tracking of adverse reactions through social media. A big data-based technology solution is required to assess and analyse this new data set and produce insightful and robust knowledge from it. A number of initiatives have been undertaken by governments and agencies to fill the gaps and unmet needs, and a first step would be the development of guidelines or position papers. A multi-stakeholder approach seems necessary for the development of such guidelines, including at least industry, patients, regulators, academic groups, and prescribers. One of the barriers to the use of social media by drug companies is the fact that if they analyse social media – even for other purposes other than drug safety – this may generate a lot of ADR information which could go beyond their ICSR processing capabilities. However, the future of ADR reporting is data intense and is almost upon us. It’s time to arm ourselves with the technology and guidelines to maximise our understanding of the patient’s voice.
CONFLICTS OF INTEREST AND DISCLAIMERS
IMS Health produces the Nexxus™ Application Suite. No other conflicts to declare.
ACKNOWLEDGEMENTS
The authors acknowledge the assistance of Dr Pradnya Kulkarni from Trilogy Writing in the preparation of this manuscript.
REFERENCES
- Safety of medicines – A guide to detecting and reporting adverse drug reactions – why health professionals need to take action. World Health Organization [2002; cited 2015 Jan 28]. Available from: http://apps.who.int/medicinedocs/en/d/Jh2992e/#Jh2992e.
- The safety of medicines in public health programmes: Pharmacovigilance an essential tool. World Health Organization [2006; cited 2015 Jan 28]. Available from: www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharmacovigilance_B.pdf?ua=1.
- FDA adverse event reporting systems (FEARS). US Food and Drug Administration (FDA) [cited 2015 Jan 28]. Available from: www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/.
- MHRA adverse drug reactions. Medicines and Healthcare Products Regulatory Agency [cited 2015 Jan 28]. Available from: www.gov.uk/the-yellow-card-scheme-guidance-for-healthcare-professionals.
- Wikibon Blog. The big list of big data infographics [2012 July 24; cited 2015 Feb 09]. Available from: http://wikibon.org/blog/big-data-infographics.
- IACP Center for Social Media. Fun facts. International Association of Chiefs of Police [2010–2015; cited 2015 Feb 09]. Available from: http://www.iacpsocialmedia.org/.
- Wikibon Blog. A comprehensive list of big data stat- istics [2012 Aug 1; cited 2015 Feb 09]. Available from: http://wikibon.org/blog/big-data-statistics/.
- Freifeld CC, Brownstein JS, Menone CM, Bao W, Filice R, Kass-Hout T, et al. Digital drug safety surveillance: Monitoring pharmaceutical products in twitter. Drug Saf. 2014;37(5):343–50.
- McGregor F, Somner JE, Bourne RR, Munn-Giddings C, Shah P, Cross V. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34(1):46–52.
- Banerjee AK, Ingate S. Web-based patient-reported outcomes in drug safety and risk management: Challenges and opportunities? Drug Saf. 2012;35(6):437–46.
- Wu H, Fang H, Stanhope SJ. Exploiting online discussions to discover unrecognized drug side effects. Methods Inf Med. 2013;52(2):152–9.
- Mao JJ, Chung A, Benton A, Hill S, Ungar L, Leonard CE, et al. Online discussion of drug side effects and discontinuation among breast cancer survivors. Pharmacoepidemiol Drug Saf. 2013;22(3):256–62.
- Vaughan Sarrazin MS, Cram P, Mazur A, Ward M, Reisinger HS. Patient perspectives of dabigatran: Analysis of online discussion forums. Patient. 2014;7(1):47–54.
- Abou Taam M, Rossard C, Cantaloube L, Bouscaren N, Roche G, Pochard L, et al. Analysis of patients’ narratives posted on social media websites on benfluorex’s (Mediator®) withdrawal in France. J Clin Pharm Ther. 2014;39(1):53–5.
AUTHOR INFORMATION
Massoud Toussi is pharmacoepidemiology and drug safety lead for North Europe and Africa in IMS Health. Massoud is medical doctor with an MSc, a PhD and an MBA. He is an active member of the ISPE, ENCePP and EUPHA. His interests are pharmacoepidemiology, drug safety and the measurement of benefit-risk balance of drugs, and health technologies with a special interest in diabetes and psychiatry.
Dr Lisa Chamberlain James is a Senior Partner of Trilogy Writing & Consulting Ltd, with a special interest in drug safety and patient information. After receiving her PhD in Pathology, Lisa began her medical writing career in Cambridge in 2000. She is a member of the EMWA Educational Committee, a leader and assessor of EMWA workshops, a member of TOPRA, and a Fellow of the Royal Society of Medicine.
Sir Alasdair Breckenridge is former Chair of the MHRA in the UK and was the first Chair of the Department of Health’s Emerging Science and Bioethics Advisory Committee. He was a member of the Medical Research Council, and worked closely on several programs in the EU and WHO. Prior to the MHRA, he was Professor of Clinical Pharmacology at the University of Liverpool and Chairman of local and regional health authorities. Sir Alasdair was knighted in 2004.